Indications
AVSOLA® is indicated for: Crohn’s Disease: Reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active Crohn’s disease who have had an... Read more
AVSOLA® is indicated for: Crohn’s Disease: Reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active Crohn’s disease who have had an... Read more
AVSOLA® is highly similar to Remicade® based on a totality of evidence, with no clinically meaningful differences in safety and efficacy.
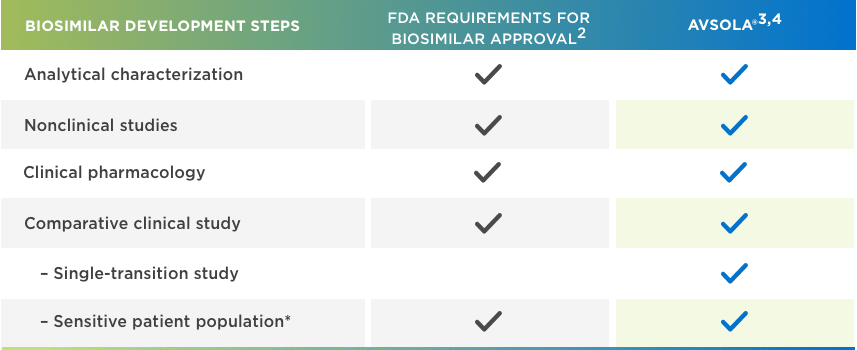
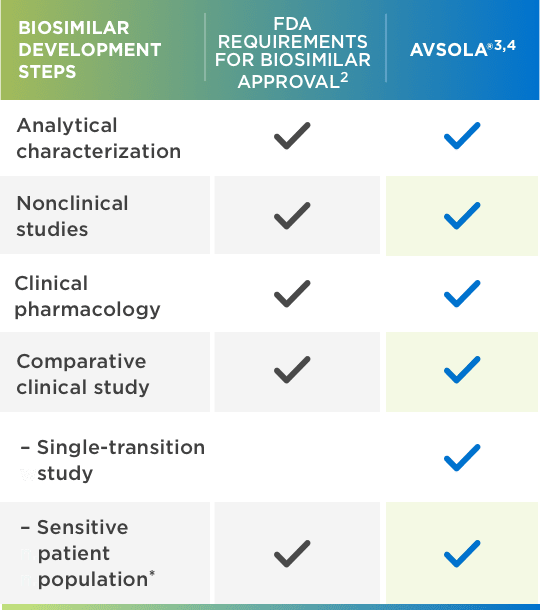
AVSOLA® is FDA approved for all Remicade® indications through extrapolation1,2,5
*Adequately sensitive to detect clinically meaningful differences between the reference product and the proposed biosimilar, should they exist.
FDA = Food and Drug Administration.
AVSOLA® IS DESIGNED TO BE HIGHLY SIMILAR TO THE ESTABLISHED CLINICAL PROFILE OF REMICADE®3
BIOSIMILARS ARE RIGOROUSLY STUDIED3,4,6
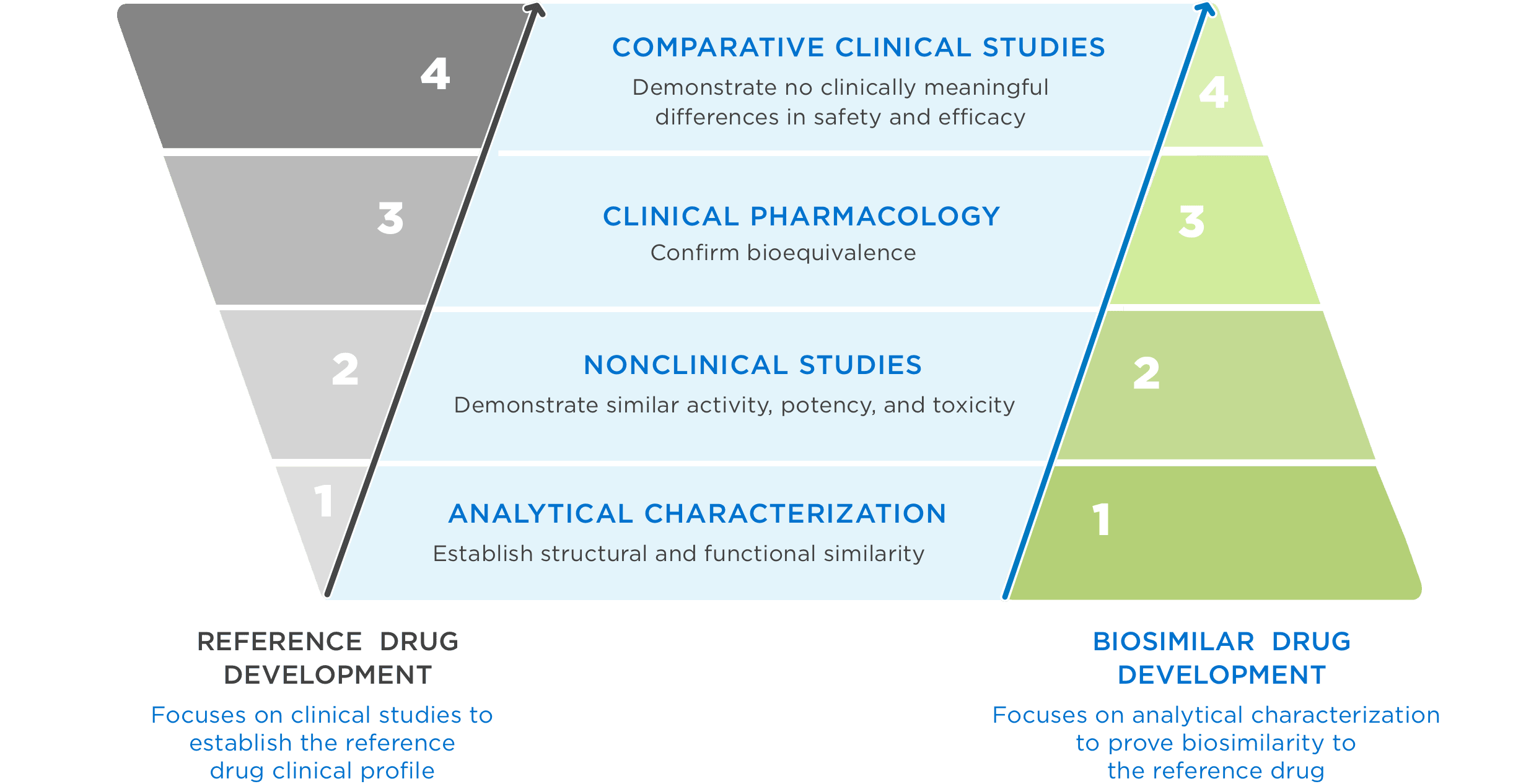
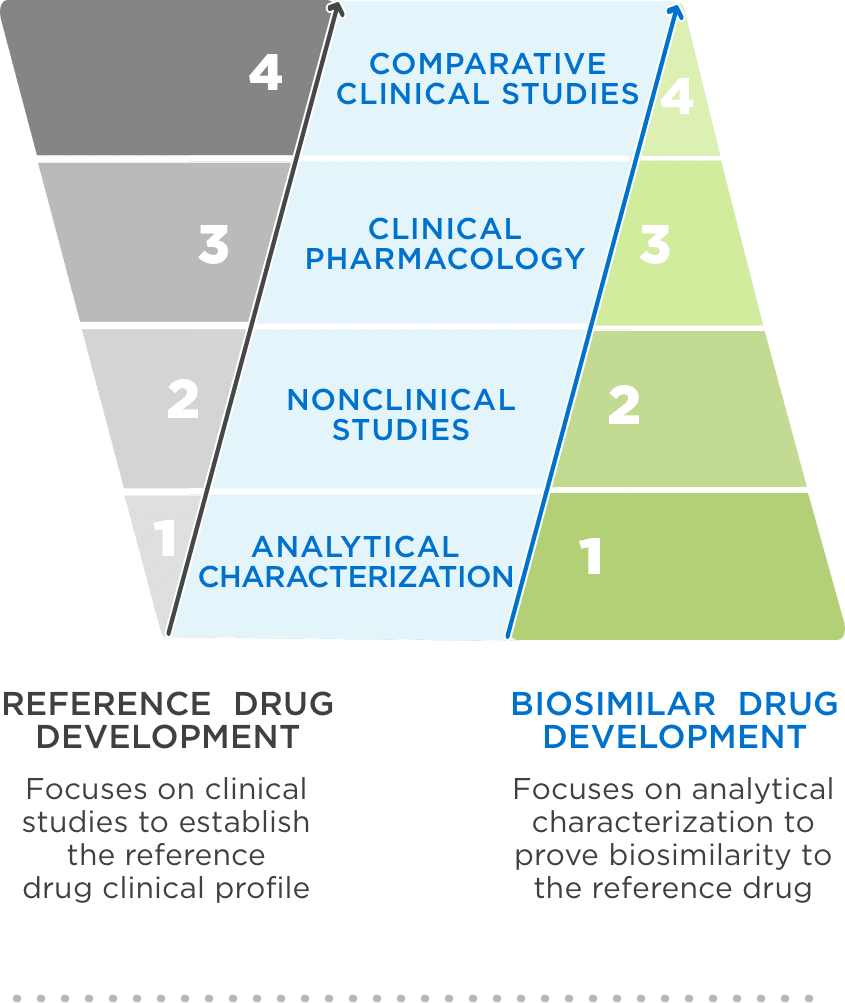
FDA approval requires a totality of evidence demonstrating no clinically meaningful differences in safety, purity, and potency2
FDA = Food and Drug Administration.
AVSOLA® COMPARATIVE STUDY DESIGNED TO EVALUATE CLINICAL SIMILARITY IN EFFICACY, SAFETY, AND IMMUNOGENICITY3
In moderate to severe RA patients
RANDOMIZED, DOUBLE-BLIND,
SINGLE-TRANSITION STUDY DESIGN
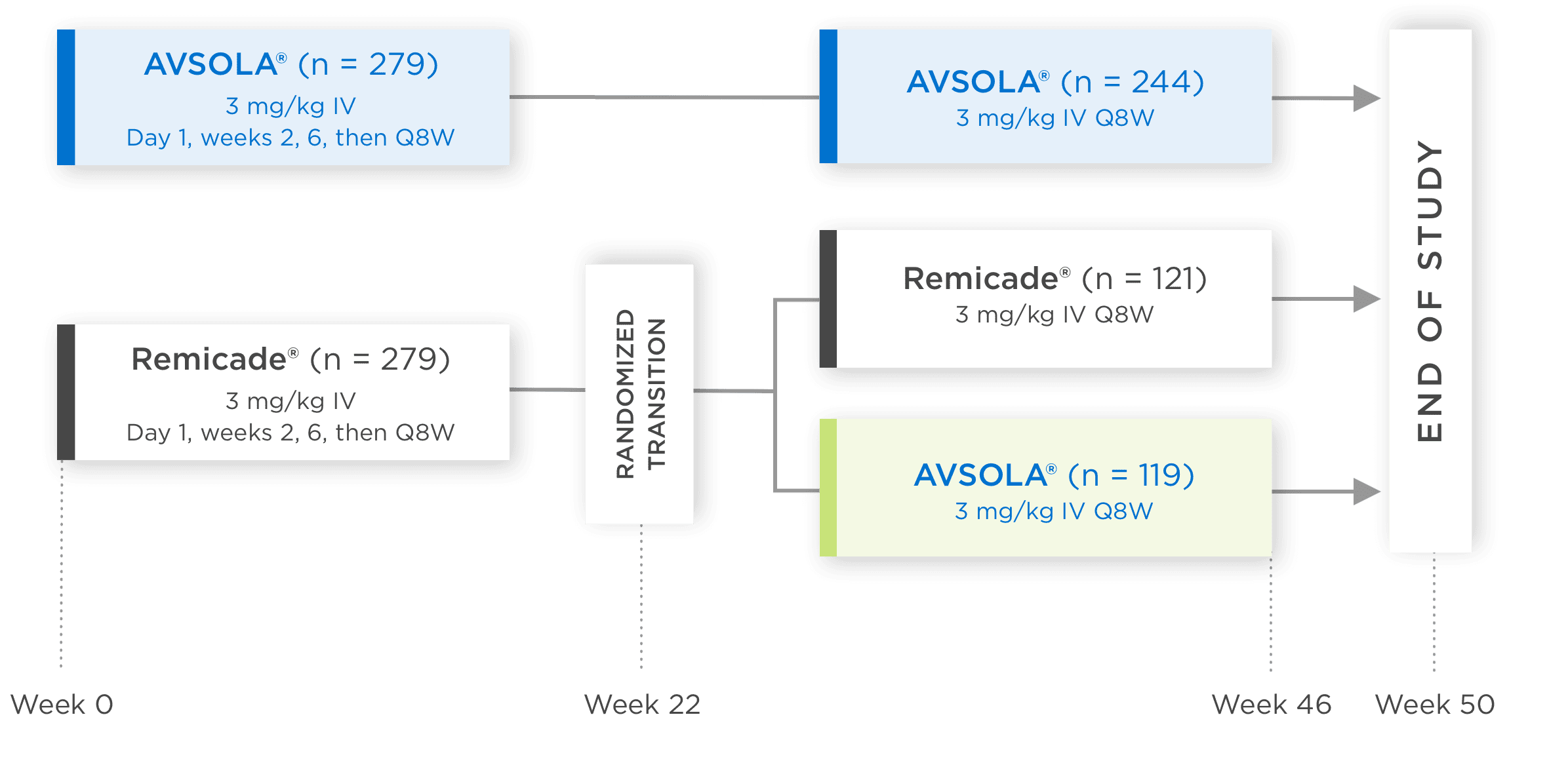
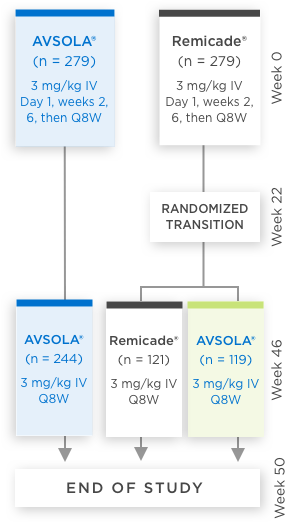
Biosimilarity was evaluated in a 50-week comparative study which included transition patients3
*Week 22 measurement pertains only to ACR50 and ACR70.
ACR20 = 20% improvement in American College of Rheumatology core set measurements; ACR50 = 50% improvement in American College of Rheumatology core set measurements; ACR70 = 70% improvement in American College of Rheumatology core set measurements; CI = confidence interval; DAS28-CRP = Disease Activity Score 28-joint count and C-reactive protein level; IV = intravenous infusion; Q8W = once every 8 weeks; RA = rheumatoid arthritis.
AVSOLA® DEMONSTRATED SIMILAR RESPONSE RATES TO REMICADE® ACROSS MULTIPLE ENDPOINTS3
PRIMARY ENDPOINT RESULT: RD of ACR20 achieved at week 22
SELECT SECONDARY ENDPOINT RESULTS
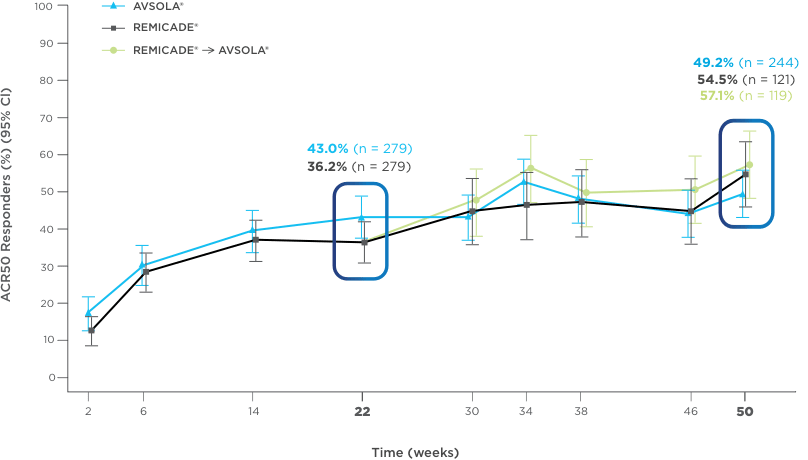
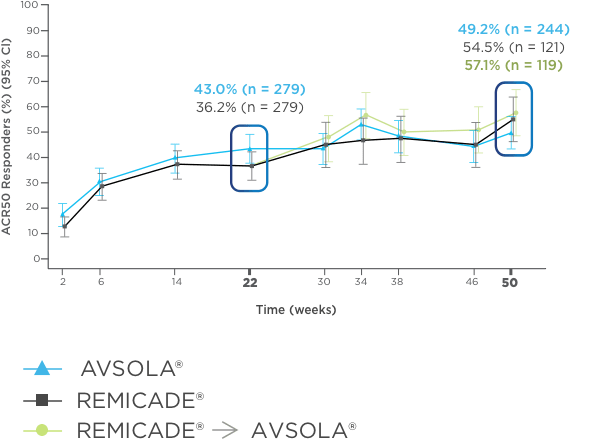
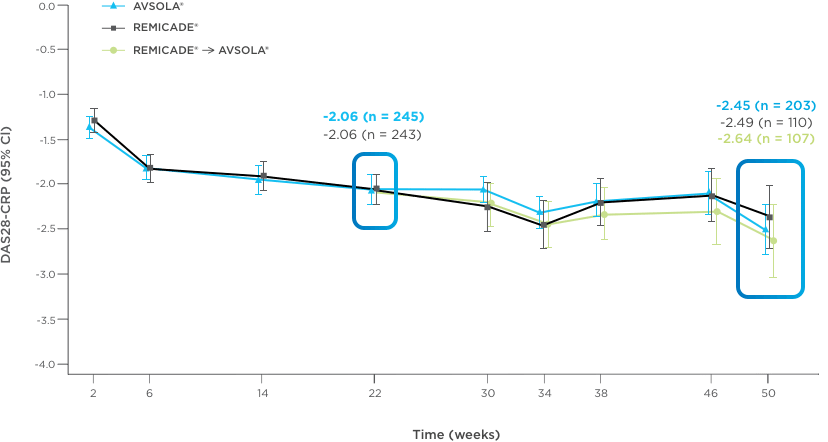
*Study in rheumatoid arthritis patients. For post week 22 summaries, only re-randomized subjects were included.
ACR20 = 20% improvement in American College of Rheumatology core set measurements; ACR50 = 50% improvement in American College of Rheumatology core set measurements; ACR70 = 70% improvement in American College of Rheumatology core set measurements; CI = confidence interval; DAS28-CRP = Disease Activity Score 28-joint count and C-reactive protein level; ITT = intent-to-treat; RD = response difference.
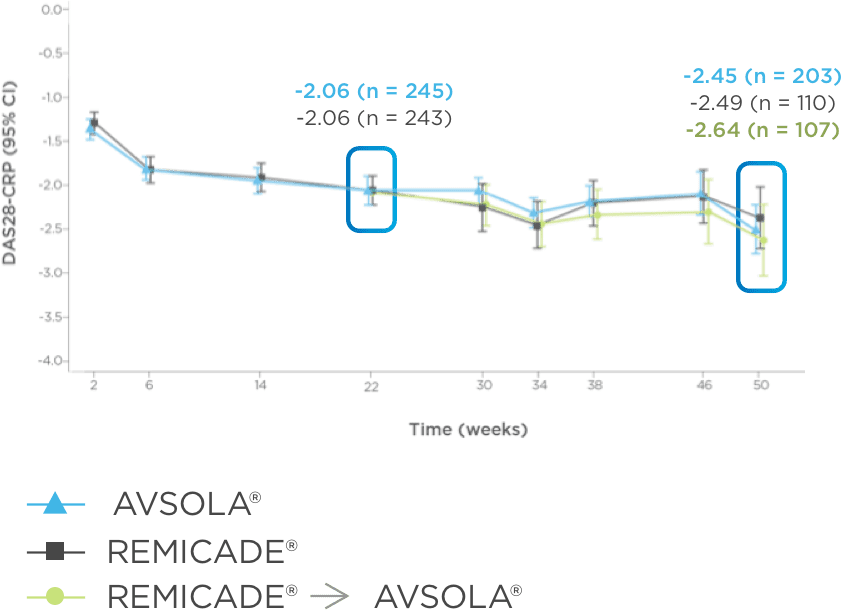
AVSOLA® CLINICAL IMMUNOGENICITY AND SAFETY ARE HIGHLY SIMILAR TO REMICADE®3,7
Including patients who transitioned from Remicade®
Neutralizing antidrug antibodies (ADAs) developed at a comparable rate3

Adverse events of interest (EOI) were comparable throughout the study3,7
AVSOLA® PHARMACOLOGY PROVEN SIMILAR IN CLINICAL STUDY4
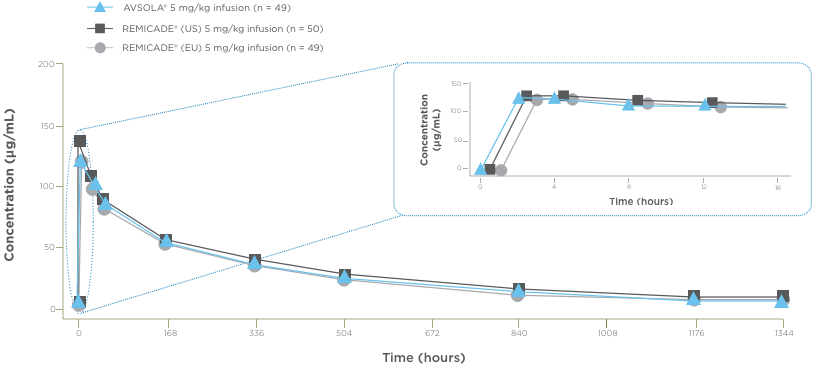
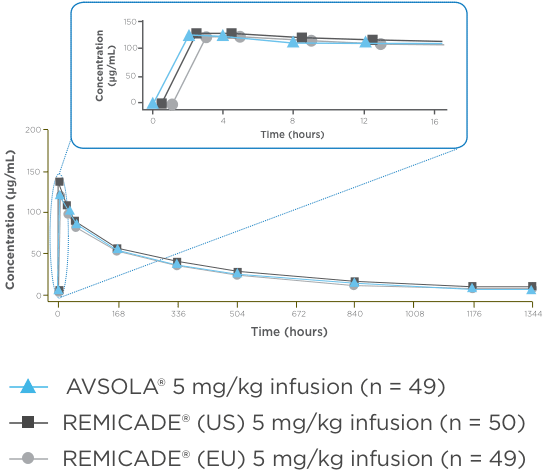
The mean serum concentration-time profiles were similar between treatments demonstrating PK similarity following single-dose administration.4
*As assessed principally by area under the serum concentration-time curve from time 0 extrapolated to infinity (AUCinf).
FDA = Food and Drug Administration.
SERIOUS INFECTIONS: Patients treated with infliximab products are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. Discontinue AVSOLA® if a patient develops a serious infection or sepsis.
Reported infections include:
The risks and benefits of treatment with AVSOLA® should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with AVSOLA®, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy, who are on treatment for latent TB, or who were previously treated for TB infection.
Risk of infection may be higher in patients greater than 65 years of age, pediatric patients, patients with co-morbid conditions and/or patients taking concomitant immunosuppressant therapy. In clinical trials, other serious infections observed in patients treated with infliximab products included pneumonia, cellulitis, abscess, and skin ulceration.
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab products. Approximately half of these cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of malignancies, including rare malignancies that are usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months after the first dose of therapy. Most of the patients were receiving concomitant immunosuppressants.
Postmarketing cases of hepatosplenic T-cell lymphoma, a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including infliximab products. These cases have had a very aggressive disease course and have been fatal. The majority of reported cases have occurred in patients with Crohn's disease or ulcerative colitis and most were in adolescent and young adult males. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF-blocker at or prior to diagnosis. Carefully assess the risks and benefits of treatment with AVSOLA®, especially in these patient types.
In clinical trials of all TNF inhibitors, more cases of lymphoma were observed compared with controls and the expected rate in the general population. However, patients with Crohn’s disease, rheumatoid arthritis, or plaque psoriasis may be at higher risk for developing lymphoma. In clinical trials of some TNF inhibitors, including infliximab products, more cases of other malignancies were observed compared with controls. The rate of these malignancies among patients treated with infliximab products was similar to that expected in the general population, whereas the rate in control patients was lower than expected. Cases of acute and chronic leukemia have been reported with postmarketing TNF-blocker use. As the potential role of TNF inhibitors in the development of malignancies is not known, caution should be exercised when considering treatment of patients with a current or a past history of malignancy or other risk factors such as chronic obstructive pulmonary disease (COPD).
Melanoma and Merkel cell carcinoma have been reported in patients treated with TNF-blocker therapy, including infliximab products. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
A population-based retrospective cohort study found a 2- to 3-fold increase in the incidence of invasive cervical cancer in women with rheumatoid arthritis treated with infliximab compared to biologics-naïve patients or the general population, particularly those over 60 years of age. A causal relationship between infliximab products and cervical cancer cannot be excluded. Periodic screening should continue in women treated with AVSOLA®.
The use of AVSOLA® at doses >5 mg/kg is contraindicated in patients with moderate or severe heart failure. AVSOLA® is contraindicated in patients with a previous severe hypersensitivity reaction to infliximab or any of the inactive ingredients of AVSOLA® or any murine proteins (severe hypersensitivity reactions have included anaphylaxis, hypotension, and serum sickness).
TNF inhibitors, including infliximab products, have been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers. Some cases were fatal. Patients should be tested for HBV infection before initiating AVSOLA®. For patients who test positive, consult a physician with expertise in the treatment of hepatitis B. Exercise caution when prescribing AVSOLA® for patients identified as carriers of HBV and monitor closely for active HBV infection during and following termination of therapy with AVSOLA®. Discontinue AVSOLA® in patients who develop HBV reactivation and initiate antiviral therapy with appropriate supportive treatment. Exercise caution when considering resumption of TNF-blocker therapy and monitor patients closely.
Severe hepatic reactions, including acute liver failure, jaundice, hepatitis, and cholestasis have been reported in patients receiving infliximab products postmarketing. Some cases were fatal or required liver transplant. Aminotransferase elevations were not noted prior to discovery of liver injury in many cases. Patients with symptoms or signs of liver dysfunction should be evaluated for evidence of liver injury. If jaundice and/or marked liver enzyme elevations (eg, ≥ 5 times the upper limit of normal) develop, AVSOLA® should be discontinued, and a thorough investigation of the abnormality should be undertaken.
In a randomized, placebo-controlled study in patients with moderate or severe heart failure (NYHA Functional Class III/IV), higher mortality rates and a higher risk of hospitalization were observed at Week 28 at a dose of 10 mg/kg and higher rates of cardiovascular events were observed at both 5 mg/kg and 10 mg/kg. There have been postmarketing reports of new onset and worsening heart failure, with and without identifiable precipitating factors. Patients with moderate or severe heart failure taking infliximab (≤5 mg/kg) or patients with mild heart failure should be closely monitored and treatment should be discontinued if new or worsening symptoms appear.
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have been reported in patients receiving infliximab products. The causal relationship to infliximab product therapy remains unclear. Exercise caution in patients who have ongoing or a history of significant hematologic abnormalities. Advise patients to seek immediate medical attention if they develop signs and symptoms of blood dyscrasias or infection. Consider discontinuation of AVSOLA® in patients who develop significant hematologic abnormalities.
Infliximab products have been associated with hypersensitivity reactions that differ in their time of onset. Anaphylaxis, urticaria, dyspnea, and hypotension have occurred in association with infusions of infliximab products. Medications for the treatment of hypersensitivity reactions should be available.
Serious cerebrovascular accidents, myocardial ischemia/infarction (some fatal), hypotension, hypertension, and arrhythmias have been reported during and within 24 hours of initiation of infliximab product infusion. Cases of transient visual loss have been reported during or within 2 hours of infusion of infliximab. Monitor patients during infusion and if a serious reaction occurs, discontinue infusion. Manage reactions according to signs and symptoms.
Agents that inhibit TNF have been associated with CNS manifestation of systemic vasculitis, seizure, and new onset or exacerbation of CNS demyelinating disorders, including multiple sclerosis and optic neuritis, and peripheral demyelinating disorders, including Guillain-Barré syndrome. Exercise caution when considering AVSOLA® in patients with these disorders and consider discontinuation if these disorders develop.
Concomitant use of AVSOLA® with anakinra, abatacept, tocilizumab, or other biologics used to treat the same conditions as AVSOLA® is not recommended because of the possibility of an increased risk of infection. Care should be taken when switching from one biologic to another, since overlapping biological activity may further increase the risk of infection.
Treatment with infliximab products may result in the formation of autoantibodies and in the development of a lupus-like syndrome. Discontinue treatment with AVSOLA® if symptoms of a lupus-like syndrome develop.
Bring patients up to date with all vaccinations prior to initiating AVSOLA®. Live vaccines or therapeutic infectious agents should not be given with AVSOLA® due to the possibility of clinical infections, including disseminated infections.
At least a 6-month waiting period following birth is recommended before the administration of any live vaccine to infants exposed in utero to infliximab products.
In clinical trials with infliximab products, the most common adverse reactions occurring in >10% of patients included infections (eg, upper respiratory, sinusitis, and pharyngitis), infusion-related reactions, headache, and abdominal pain.
AVSOLA® is indicated for:
Crohn’s Disease: Reducing signs and symptoms and inducing and maintaining clinical remission in adult patients with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy. AVSOLA® is indicated for reducing the number of draining enterocutaneous and rectovaginal fistulas and maintaining fistula closure in adult patients with fistulizing Crohn’s disease.
Pediatric Crohn’s Disease: Reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients 6 years of age or older with moderately to severely active Crohn’s disease who have had an inadequate response to conventional therapy.
Ulcerative Colitis: Reducing signs and symptoms, inducing and maintaining clinical remission and mucosal healing, and eliminating corticosteroid use in adult patients with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy.
Pediatric Ulcerative Colitis: Reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients 6 years of age and older with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy.
Rheumatoid Arthritis in combination with methotrexate: Reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis.
Ankylosing Spondylitis: Reducing signs and symptoms in adult patients with active ankylosing spondylitis.
Psoriatic Arthritis: Reducing signs and symptoms of active arthritis, inhibiting the progression of structural damage, and improving physical function in adult patients with psoriatic arthritis.
Plaque Psoriasis: The treatment of adult patients with chronic severe (i.e., extensive and/or disabling) plaque psoriasis who are candidates for systemic therapy and when other systemic therapies are medically less appropriate. AVSOLA® should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician.
Please see full Prescribing Information.
AVSOLA® is a registered trademark of Amgen, Inc.
Important Safety Information
SERIOUS INFECTIONS:
Patients treated with infliximab products are at increased risk for developing serious infections that may lead to hospitalization or death.
Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. Discontinue AVSOLA® if a patient develops a serious infection or sepsis.
Reported infections include:
References: 1. AVSOLA® (infliximab-axxq) Prescribing Information, Amgen. 2. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. Accessed April 11, 2022. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf 3. Genovese M, Sanchez-Burson J, Oh M, et al. Comparative clinical efficacy and safety of the proposed biosimilar ABP 710 with infliximab reference product in patients with rheumatoid arthritis. Arthritis Res Ther. 2020;22:60. Published 2020 Mar 26. 4. Chow V, Oh M, Gessner M, Fanjiang G. Pharmacokinetic similarity of ABP 710, a proposed biosimilar to infliximab: results from a randomized, single-blind, single-dose, parallel-group study in healthy subjects. Clin Pharmacol Drug Dev. 2020:246-255. doi:10.1002/cpdd.738 5. Remicade® (infliximab) Prescribing Information, Janssen Biotech. 6. Saleem R, Cantin G, Wikström M, et al. Analytical and function similarity assessment of ABP 710, a biosimilar to Infliximab reference product. Pharm Res. 2020;37:114. doi.org/10.1007/s11095-020-02816-w" 7. Data on file, Amgen; [CSR20140111, 2019].